Industrial production of substances that impart the umami taste.
Author:
Hellen Dea Barros Maluly (MALULY, H.D.B.)
Pharmacist and PhD in Food Science. This CV can be accessed at: http://lattes.cnpq.br/2754275781355863
Published on: 2 de July de 2021
Abstract
Products on the market that contain umami taste, such as monosodium glutamate, are produced through the fermentation process. Learn about the main steps involved in the industrial production of these substances.
Palavras-chaves: taste, flavor, glutamate, monosodium glutamate, umami, glutamic acid
Chemist Kikunae Ikeda was considered one of the greatest scientists in Japanese history. This is because he studied the properties of glutamic acid, an amino acid isolated by Ritthausen in 1866 and molecularly identified by Wolff in 1890 (Vickery & Schmidt, 1931). Ikeda hypothesized that this substance could provide what he called the fifth basic taste, umami, and that it could also help improve the Japanese diet, which was poor in complex proteins.
After isolating the amino acid from kombu seaweed, the researcher began a series of investigations until he developed a substance that could be used in food as a type of seasoning. Initially, it was not possible to extract the amino acid directly from kombu seaweed, as it would require tons of it for industrial-scale production. So he used gluten, the main protein in wheat, as the primary source, since this protein is very rich in glutamic acid. The process began with acid hydrolysis, which broke down the protein and generated free glutamic acid, which was neutralized and transformed into salts (Illustration 1).
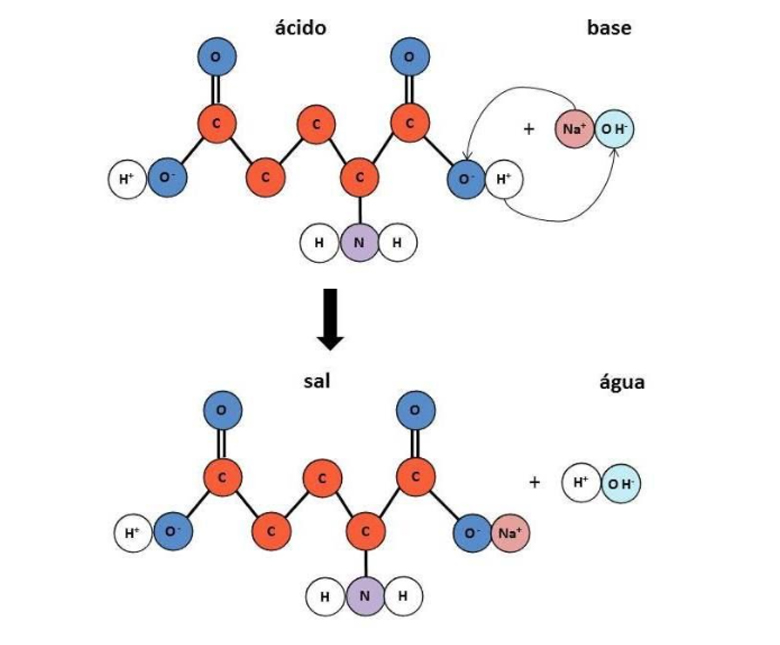
Glutamic acid neutralization reaction
The patent for the production of monosodium glutamate (MSG) was soon granted to the researcher. From that moment on, the industrial-scale production process was authorized and initiated by Ikeda and entrepreneur Saburousuke Suzuki, who together founded the company Ajinomoto® in 1909 (Sano, 2009).
However, research to improve the substance’s production process was still underway, as there were still some difficulties in extracting glutamic acid from gluten, particularly regarding production demand. For these reasons, various methods were developed, but it wasn’t until the 1950s that the technology best suited for large-scale production was established: direct fermentation (the transformation of a raw material into another product by microorganisms—bacteria or yeast). Fermentation processes are widely used in the food industry, particularly for the production of bread, yogurt, and beverages such as wine and beer, and also in the pharmaceutical industry for the production of medicines. For the microorganisms involved in fermentation to multiply, substrates are required—that is, energy sources such as macronutrients, especially carbohydrates.
In the case of fermentation to produce the substances that impart umami flavor (monosodium glutamate, inosinate, and disodium guanylate), harmless microorganisms use sugars from plant-based raw materials such as sugarcane, beets, cassava, and corn, as well as other sources that can provide energy for their multiplication (such as nitrogen sources, for example). As a result of fermentation, there is an intense production of glutamic acid and/or nucleotides, which are neutralized through an acid-base reaction so that these substances can bind to ions and thus form salts (acid + base = salt + water). Most often, the ion most commonly used to form salts is sodium, but others, such as potassium, are also present. From this point on, the purification process begins to remove fermentation residues, drying, and finalize the process.
All substances that impart umami taste undergo rigorous quality control processes to verify their purity and ensure they can be used in the food industry in the best possible way. All this to add a hint of umami to our foods and transform meals into a great sensory experience!
References
- Vickery HB, Schmidt CLA. The history of the discovery of the aminoacids. Chem Rev. (1931) 9: 169-318.
- Sano C. History of glutamate production. Am J Clin Nutrit 2009, 90 (3S): 728S-732S.




